New-onset diabetes after transplantation (nodat) and glucose Metabolism in at-risk patients receiving cyclosporine versus tacrolimus: A randomised controlled trial
Neeraj Jain1, Manish rathi2, Ashish Sharma3.
1Nephrology, NSCB Medical College, Jabalpur, India; 2Nephrology, PGIMER, Chandigarh, Chandigarh, India; 3Transplant Surgery, PGIMER, Chandigarh, Chandigarh, India
Primary objective:
1) Incidence of “New-onset diabetes after transplant (NODAT)” in high-risk patients
who have received tacrolimus versus cyclosporine.
Secondary objectives:
1) FBS, OGTT, and HbA1c in high-risk patients who have received cyclosporine versus tacrolimus.
2) Insulin sensitivity (%S), insulin resistance (IR) and beta cell function (%B) in high risk patients who have received cyclosporine versus tacrolimus.
3) Incidence of acute rejections in patients who have received cyclosporine versus tacrolimus.
Study protocol
This study was an open-labeled, randomized, prospective pilot study. Patients with risk factor defined in inclusion criteria were randomized to receive cyclosporine and tacrolimus and were followed in post-transplant period for the development of NODAT and assessed for altered glucose metabolism and insulin parameters using HOMA-2 (homeostatic model
assessment) calculator.
Patient enrollment
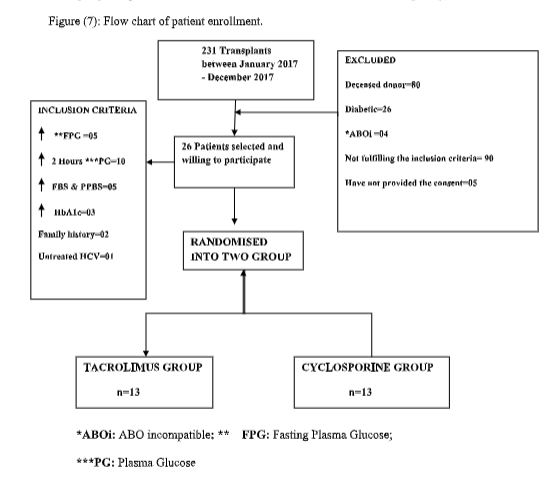
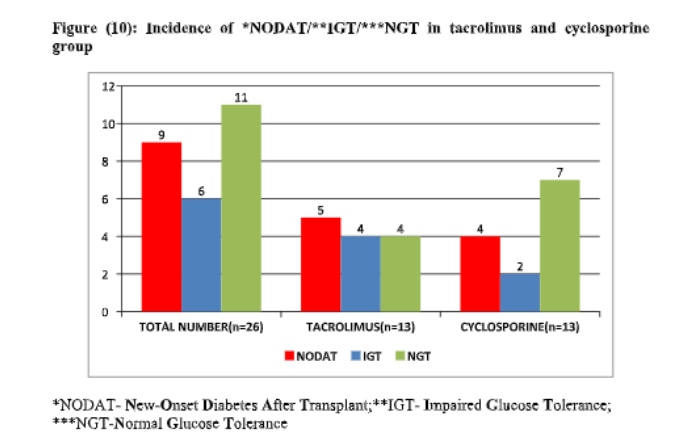
Result
Conclusion
1. The incidence of NODAT or pre-diabetes at 6 months post-transplant was lower with cyclosporine than with tacrolimus without a significant difference in short-term outcomes.
2. Cyclosporine has shown to have relative better β cell function as compared to tacrolimus and can be considered in at-risk patients for NODAT with low immunological risk, though in this study cyclosporine has not prevented the occurrence of NODAT but overall glucose metabolism parameters favours its use this subset of patients and is non-inferior to tacrolimus in preventing graft rejection.
3. As the sample size of our study is small and duration of follow up was also short, findings need to be corroborated in the study with sufficient sample size before any conclusion can be drawn.
