Oral pH-modified release budesonide for preventing recurrence of IgA nephropathy in kidney transplant recipients: A single center experience
Bogdan Sorohan1,2, Dorina Tacu1, Cristina Bucșa1, Corina Țincu1, Paula Vizireanu1, Elena Cristoiu-Strachinariu1, Ioanel Sinescu1,3, Cătălin Baston1,3.
1Kidney Transplantation, Fundeni Clinical Institute, Bucharest, Romania; 2Nephrology, "Carol Davila" University of Medicine and Pharmacy, Bucharest, Romania; 3Urology, "Carol Davila" University of Medicine and Pharmacy, Bucharest, Romania
Introduction: Recurrent IgA nephropathy (IgAN) after kidney transplantation (KT) is associated with a higher risk of graft loss. There are no known preventive therapies for IgAN recurrence so far. Oral budesonide has demonstrated promising results in patients with IgAN on native kidneys, but studies on recurrence management after KT are scarce and dissonant. We sought to evaluate the effect of oral pH-modified release budesonide in preventing recurrence of IgAN in KT recipients.
Methods: We performed a single-arm, open-label, prospective study of 19 adult KT recipients (KTR) with biopsy-proven IgAN on native kidneys, enrolled between 2021 and 2023. Median follow-up period was 15 months (10-22). All patients received 3mg of oral pH-modified release budesonide from day 45 after KT. Primary endpoint was clinically significant and biopsy-proven IgAN recurrence. Graft biopsy has been performed at indication. Secondary outcomes were: mean change of estimated glomerular filtration rate (eGFR) from baseline, proteinuria ≥ 0.5 g/24h, hematuria, creatinine increase ≥ 25% from baseline, incidence o graft loss, incidence of rejection and drug tolerability.
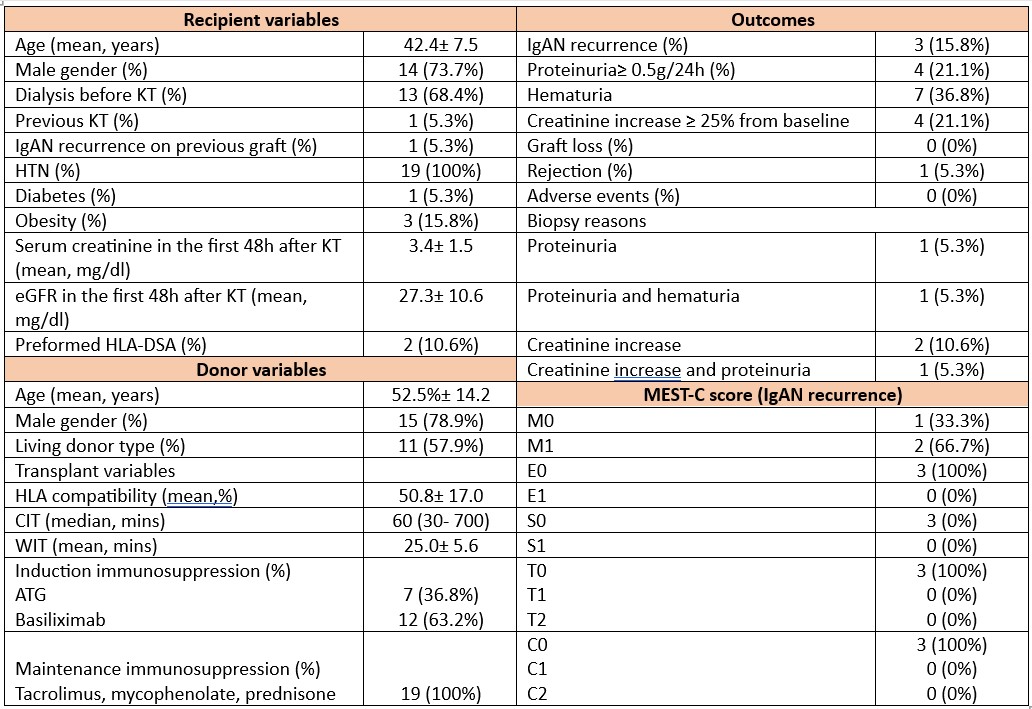
Results: Baseline characteristics are summarized in Figure 1. Mean age of KTR was 42.4± 7.5 years, 73.7% were males and 68.4% performed hemodialysis before KT. 31.6% of patients has crescents on native biopsies, 38.9% had IgA/C3 codominance of immunofluorescence and 84.2% received immunosuppression. Mean donor age was 52.5± 14.2 years and 57.9% of KTR received a graft from a living donor. Basiliximab induction was used in 36.8% of cases. All patients received maintenance immunosuppression with tacrolimus, mycophenolate and prednisone. Mean eGFR at baseline was 27.3± 10.6 ml/min/1.73m2. Among 19 patients 5 graft biopsies have been performed based on clinical manifestations. The incidence of biopsy-proven IgAN recurrence was 15.8%. Based on MEST-C score all cases were mild forms. Mean time of recurrence was 14.7±8.0 months. Mean eGFR change from baseline to last follow-up was +41.31± 21.64 (95%CI: 30.88- 51.74) (Figure 2). Proteinuria≥ 0.5 g/24h was observed in 21.1% of KTR, hematuria in 36.8% and creatinine increase ≥ 25% from baseline in 21.1% of cases. One patient developed rejection. No cases of graft loss have been noted. No adverse effects related to budesonide have been reported. No significant difference was found between patients with and without IgAN recurrence in terms of mean eGFR change from the moment of recurrence to last follow-up [adjusted mean difference = +1.43 mL/min/1.73 m2 (95% CI: −21.54 to +24.42, p = 0.89)] (Figure 2).
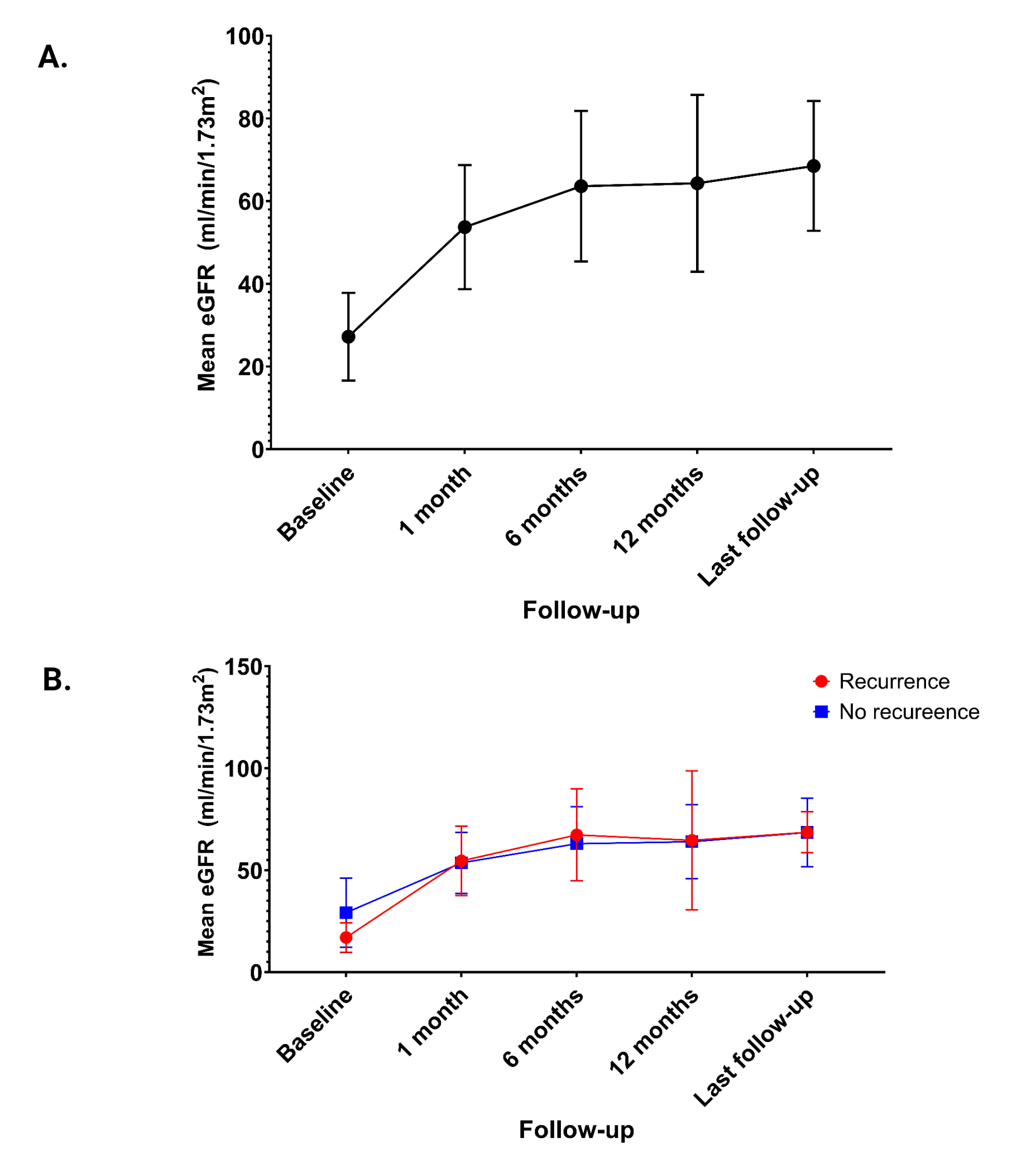
Conclusion: IgAN recurrence after KT occurred in 15.8% of patients under preventive treatment with oral pH-modified release budesonide. No difference regarding eGFR change was observed between patients with and without recurrence. None of the patients experienced any adverse events.
[1] IgA nephropathy
[2] Kidney transplantation
[3] recurrence
[4] eGFR
[5] proteinuria
[6] hematuria
[7] budesonide
[8] biopsy
