Long-term survival in pig-to-NHP kidney xenotransplantation with clinically relevant calcineurin inhibitor-based immunosuppression
Daniel Eisenson1, Yu Hisadome1, Hayato Iwase1, Michelle Santillan1, WeiLi Chen1, Alexander Schulick1, Du Gu1, Daniel Warren1, Lars Burdorf2, David Ayares2, Andrew Cameron1, Kazuhiko Yamada1.
1Johns Hopkins University, Baltimore, MD, United States; 2United Therapeutics Corporation, Blacksburg, VA, United States
Introduction: Xenotransplantation is an increasingly promising solution to the organ shortage crisis with recent studies from our institution demonstrating long-term survival in pig-to-NHP kidney xenotransplantation. However, no studies to date have demonstrated long-term solid organ xenograft survival without CD40/CD154 costimulatory blockade, and there are currently no FDA-approved agents associated with this pathway. The divide between effective immunosuppression regimens for human allotransplantation and NHP xenotransplantation raises concerns for the clinical translation of preclinical NHP data.
Methods: Six baboon recipients underwent porcine kidney xenotransplantation using 10GE source pigs (n=5 recipients) and GalTKO source pigs (n=1 recipient). All recipients were deemed low-risk for hyperacute graft loss as assessed by our preformed natural antibody screening algorithm. Recipients were induced with rituximab and thymoglobulin, and then received maintenance immunosuppression which featured only clinically available, FDA-approved agents: mycophenolate mofetil from post-operative day (POD) -5, tacrolimus from POD -1, belatacept (CTLA4-Ig) on POD 2 (continued weekly), methylprednisolone at low dose initiated on POD 6, and sirolimus initiated on POD 42. Both native baboon kidneys were removed at the time of transplantation. This analysis assessed outcomes among recipients who maintained therapeutic (10-15 ng/mL) tacrolimus levels versus subtherapeutic (<10 ng/mL) tacrolimus levels.
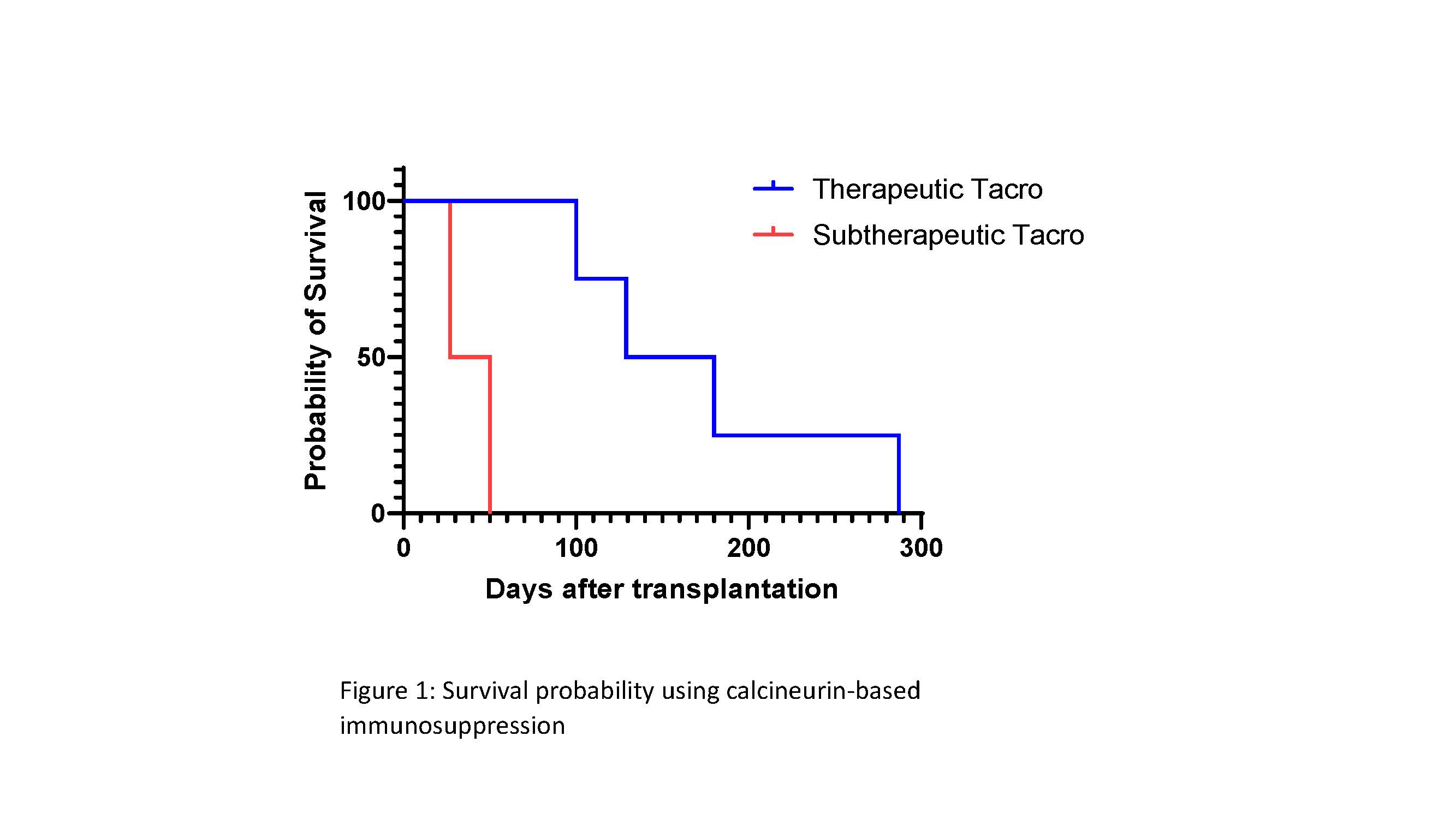
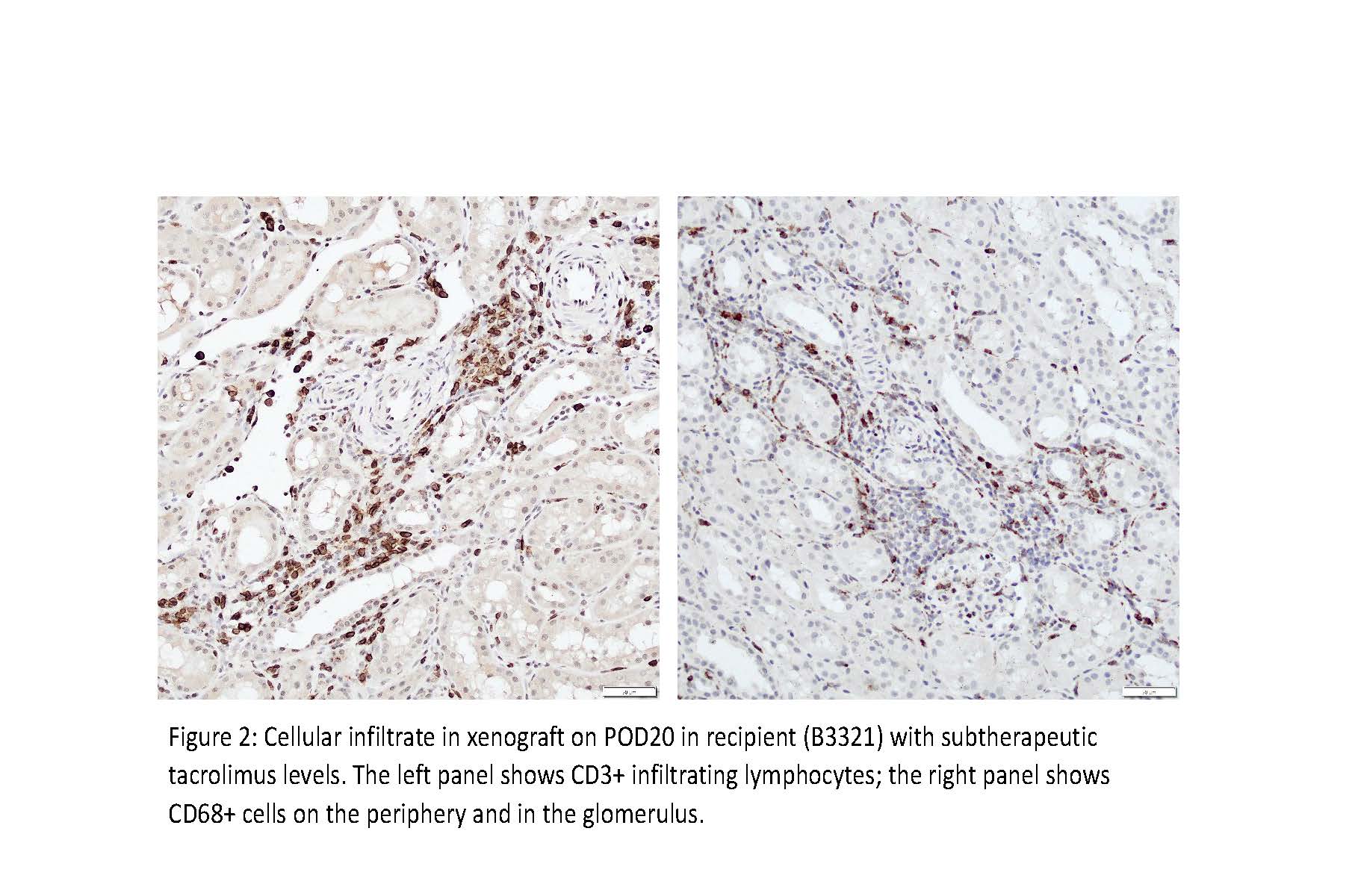
Results: Xenografts were life-supporting for 285, 180, 129, 100, 50, and 27 days after transplantation (overall mean 128.5 days and median 114.5 days). Four recipients with therapeutic tacrolimus levels (10-15 ng/ml in the first month and tapering to 5-10 ng/ml) maintained stable graft function for >3 months (median 154.5 days and mean 173.5 days). Two recipients with subtherapeutic tacrolimus blood levels experienced early rejection episodes within two months (mean 38.5 days). Biopsies from xenografts in these two recipients showed lymphocyte and macrophage infiltrate in the first 30 days.
Conclusion: This study provides the first evidence that conventional, FDA-approved immunosuppression regimens can be used to achieve long-term xenograft survival in pig-to-NHP solid organ transplantation. These data help to resolve an important discrepancy between preclinical pig-to-NHP xenotransplantation data and the clinical allotransplantation experience, and point to a possible explanation – the challenges of maintaining therapeutic calcineurin inhibitor levels – as to why previous studies have failed to demonstrate survival >30 days.
This study was supported by United Therapeutics Corporation.
[1] xenotransplantation, kidney transplantation, tacrolimus, conventional immunosuppression, baboon, clinically relevant
