10GE pig-to-baboon lung xenotransplantation, combined with immunosuppressive therapy and pharmacologic treatment
Sara De Taeye1, Sho Takemoto1, Seyedamir Sanatkar1, Lars Burdorf2, Zahra Habibabady1, Akihiro Maenaka1, Kohei Kinoshita1, Ikechukwu Ileka1, Victoria Diaz1, Maddie Ma1, Shannon Pratts1, David Ayares2, Richard N Pierson III1.
1Division of Cardiac Surgery, Department of Center for Transplantation Sciences, Massachusetts General Hospital, Charlestown, MA, United States; 2Revivicor Inc., Blacksburg, VA, United States
Introduction: Xenotransplantation holds promise as a solution to critical shortage of donor organs, provided effective immunosuppression can be achieved. Previously reported lung ex vivo pig-to-human and in vivo pig-to-baboon xenotransplants have demonstrated variation in survival attributed to donor gene modifications (GE). This study aims to evaluate life-supporting function of 10GE-lungs, combined with selective innate and adaptive immune suppression.
Methods: Left single lung transplants (n=2) were conducted in 25kg baboons utilizing 10GE pigs: lacking 1,3Gal.β4Gal.Neu5Gc (triple knockout; TKO) and with additional modifications GHKO.hCD46.hCD55.hCD47.hHO-1.hEPCR.hTBM. Lung donors were vWF-depleted through DDAVP administration. Recipients underwent splenectomy one week before induction therapy with B-cell (αCD20) and T-cell depletion (rATG), along with CVF administration. Treatment included selectin and integrin blockers, αGP1b, 1-BIA, antihistamines, corticosteroids, α1antitrypsin, αCD154 and C1esterase, and IL-6, IL-8, TNFα pathway inhibition. Two hours of kidney preperfusion preceded the lung xenotransplant in case 10523. Heparin and CD154-based immunosuppression were administered until end of study.
Results: Survival times were comparable to previously reported in vivo lung transplantation (figure 1). B10423 was extubated with good lung function at 44h post-transplant, but experienced sudden oxygen saturation decline at 60h, prompting study termination. B10523 developed increasingly sanguinous secretions from the xenograft within 14h. Donor specific IgG and IgM antibody (figure 2A) decreased following splenectomy and induction treatment with αCD20 and rATG, whereas renal preperfusion (right panel, pre vs -5) and lung xenograft revascularization (-5 versus subsequent) were associated with modest further decrease in anti-donor antibody titers. Day zero histology showed erythrocyte congestion in pulmonary alveolar capillaries in both cases and intravascular microthrombi in B10423. At necropsy, loss of vascular barrier function was manifest as alveolar flooding of dependent lung in both xenografts (figure 2B); inflammation of the native right lung was also observed in the longer-surviving recipient.
Conclusion: Using a pharmacologic cocktail designed to inhibit known mechanisms of lung xenograft injury, 10GE pig lungs, with or without pre-transplant kidney adsorption, exhibit survival similar to lungs with fewer gene edits, and shorter than the median survival obtained with h*pvWF modification on a simpler (GTKO.hCD46) background. Based on this preliminary data, we conclude that adding protection from NK cells by HLA-E expression to this 10GE model, and perhaps humanizing vWF or omitting the CMAHKO, may be necessary to achieve consistently prolonged pig lung xenograft survival in baboons.
" href="https://app.tts2024.org/papers/body/1660#">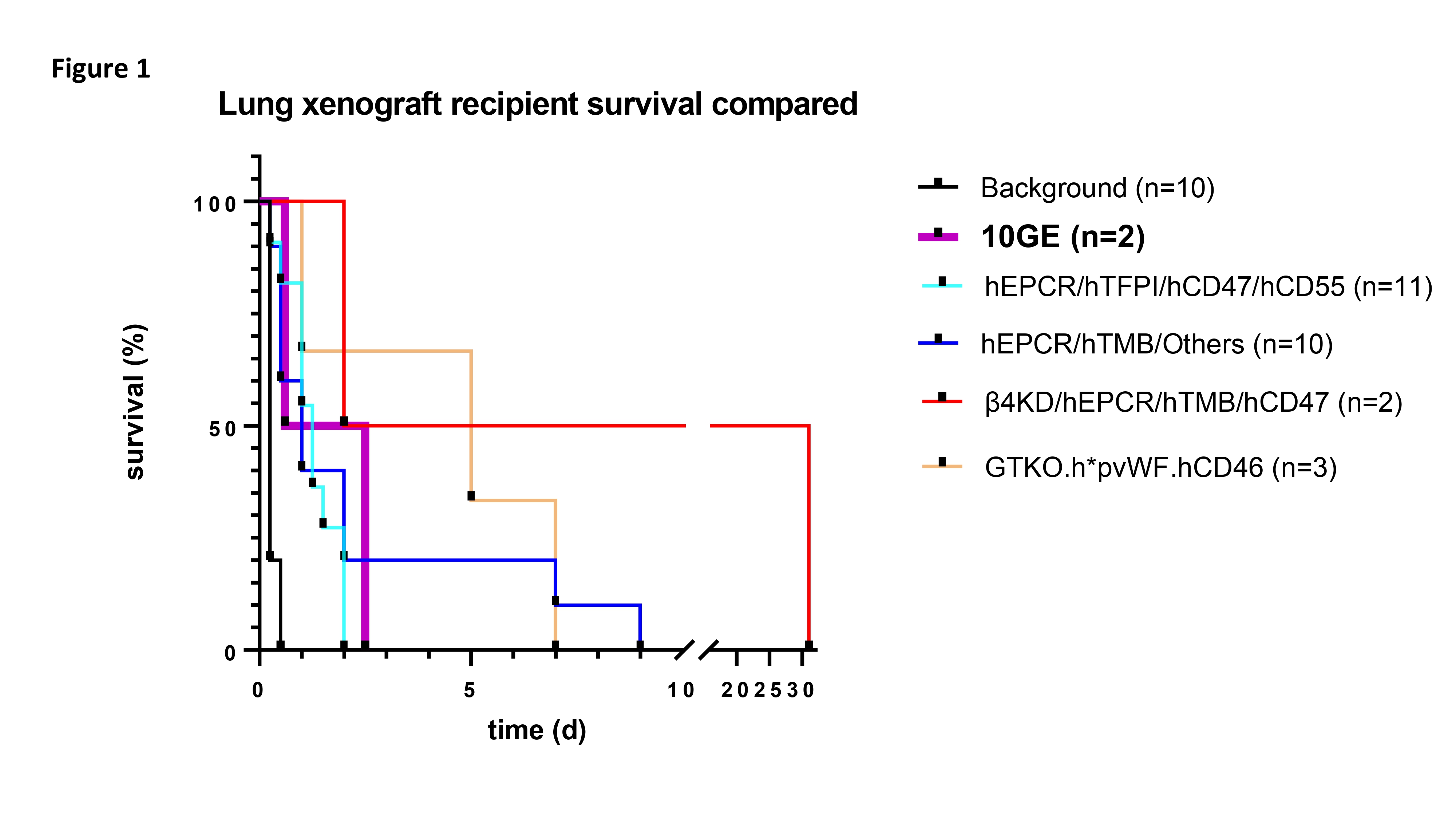
" href="https://app.tts2024.org/papers/body/1660#">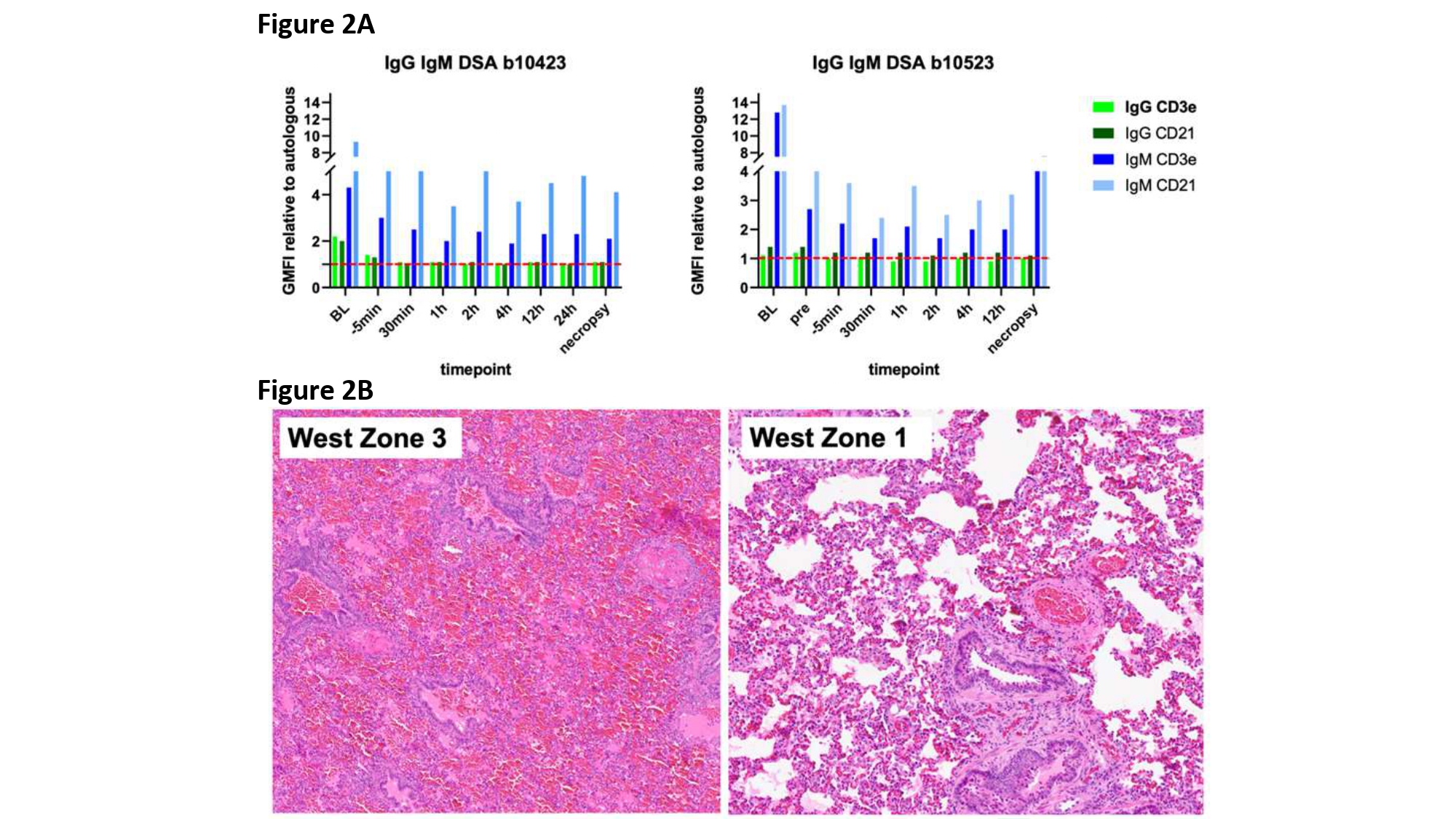
Sara De Taeye is supported by the Belgian American Educational Foundation (BAEF) as Boulpaep Master's Level Research Fellow.
[1] 10-gene modified pig
[2] immunosuppression
[3] xenotransplantation
[4] lung transplantation
