Outcomes of mTOR Inhibitors switch for cutaneous and/or anogenital warts among kidney transplant recipients
Cansu Erel Gezegen1, Rabia Hacer Hocaoglu2, Seda Safak Ozturk2, Nurana Guller2, Ahmet Burak Dirim2, Ayse Serra Artan2, Erol Demir2, Halil Yazici2, Aydin Turkmen2, Ozgur Akın Oto2.
1Department of Internal Medicine, Istanbul Training and Research Hospital, Istanbul, Turkey; 2Division of Nephrology, Department of Internal Medicine, Istanbul Medical Faculty of Istanbul University, Istanbul, Turkey
Introduction: Warts are common among kidney transplant recipients (KTR) due to immunosuppressive treatments, impacting quality of life and treatment adherence. Cutaneous warts (CW) are linked with skin tumors, and genital warts (GW) with anogenital malignancies. Mammalian target of rapamycin inhibitors (mTORi) show promise in treating warts due to their antiviral and antitumoral effects.
Method: Between January 1976 and October 2022, 1813 files were examined in the archive of Istanbul University Faculty of Medicine Kidney Transplantation Centre. Patients aged 18 years and older with CW and/or GW were included. KTR who were lost to follow-up and/or had incomplete records, as well as warts that occurred after graft failure, were excluded. Characteristics of KTR, transplantation data, laboratory values, and accompanying lesions were recorded. Patients were categorized into three groups: mTORi (+) Warts: patients who switched from calcineurin inhibitors (CNI) to mTORi due to warts ± anogenital premalignant lesions, mTORi (+) Others: patients who switched from CNI to mTORi due to skin tumors or other malignancies, and mTORi (-): patients who did not switch from CNI to mTORi. In this study, the primary outcome of switching to mTORi in treating warts is graft loss, while secondary outcomes such as wart response, rejection and patient survival are analyzed.
Results: Out of the 140 patients, 52% were women, with a mean age of 49 ± 11.5 years. The mean follow-up was 184 ± 89 months. The prevalence of warts was 8.8%. CW were observed in 92 patients (66%), and GW in 62 patients (44%). The median time from transplantation to wart occurrence was 66 months (IQR 33-134.5). In addition to warts, malignancy (15%), anogenital premalignant lesions (11%), and skin tumors (10%) were present. A switch from CNI to mTORi was performed in 55 patients (39%). The characteristics of the groups were similar except gender. GW and anogenital localization were preferred for mTORi switch. Complete and partial wart response was higher in the mTORi (+) Warts group compared to the mTORi (-) group (64% vs 32%, p = 0.02). Adverse reactions associated with mTORi developed in 82% of those who underwent the change, while they were more common in the mTORi (+) Others group. The most common adverse reactions were proteinuria (29%), hyperlipidemia (27%), and edema (20%). In the mTORi (+) Warts group, graft loss was observed in 5 patients (16%), and rejection did not occur. Kaplan-Meier Analysis indicated no significant difference between the groups in terms of graft or patient loss.
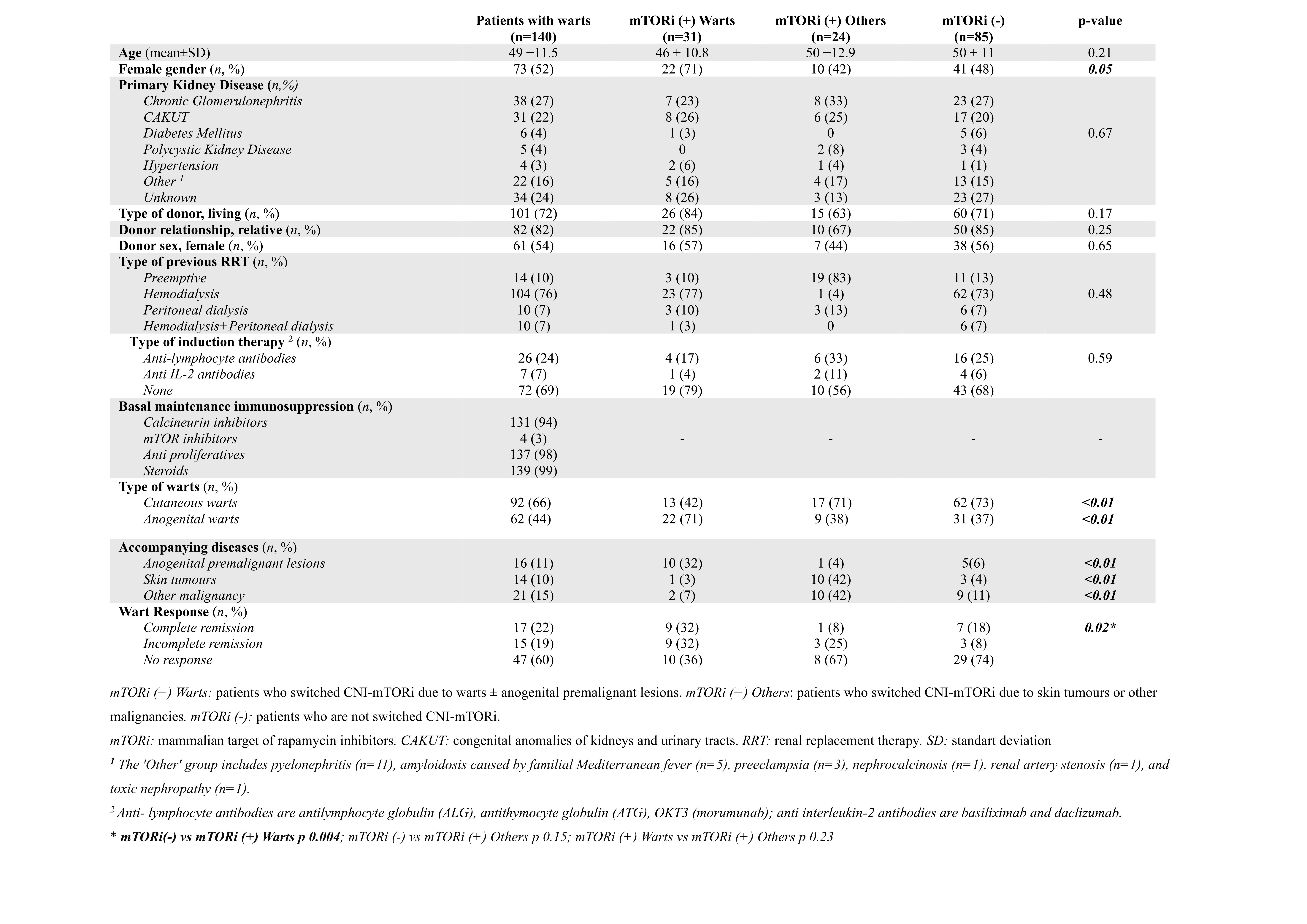
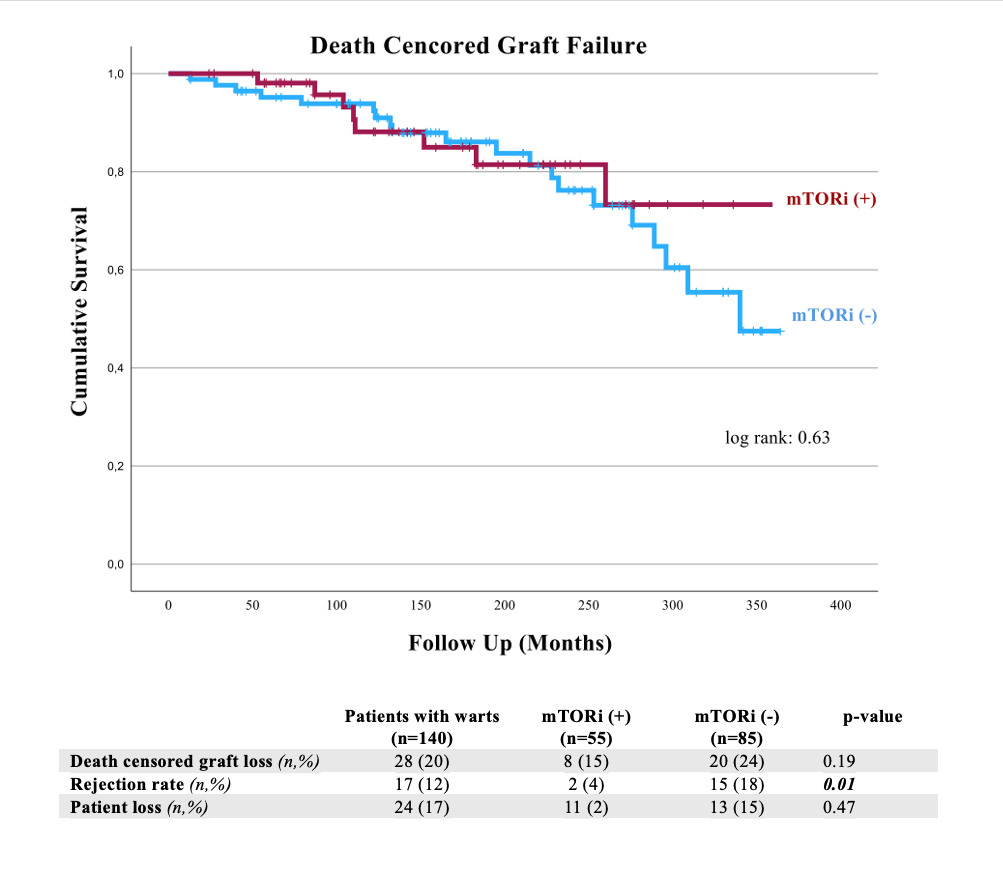
Conclusion: mTORi emerge as an effective treatment for warts in KTR, albeit its use is constrained by adverse reactions. Additionally, mTORi are considered less potent immunosuppressants and making them unfavorable for high immunological risk patients. This may explain the absence of significant differences in graft/patient loss and the low rates of rejection.
[1] Immunosuppression
[2] Graft Rejection
[3] Human papillomavirus
[4] Allograft Survival
[5] Cutaneous wart
[6] Genital wart
[7] mTOR
[8] mTOR inhibitor
[9] Mammalian Target of Rapamycin
[10] Kidney Transplantation
